How can the same gene kill you and save your life?
The gene for sickle cell disease is one such example. It is a version of the hemoglobin gene that causes sickle cell disease, and when a person has two copies of that gene version, it causes a debilitating disorder of the oxygen-carrying red blood cells. In the past, scientists were left scratching their heads as to why it persists in our population. Many wondered “shouldn’t natural selection have weeded out of our gene pool this debilitating disease?” It turns out that while sickle cell disease is deadly, it has one major advantage: people who carry even just one copy of the gene are resistant to malaria. The gene is a life-saving killer.
This is not the only example of a gene that has a long history of causing a tremendous amount of human suffering that also may have greatly improved biological fitness. Genes for Cystic fibrosis, Tay-Sachs, and even the BRCA genes that increase the risk for breast cancer persist because they offer some evolutionary benefit despite their obvious deleterious effects. We generally think of mutations to our DNA as bad, and for the most part this is true. Many novel mutations are eliminated by natural selection because they have a negative impact on a person’s fitness. But in some rare cases, a mutation is selected for because it also offers a tremendous benefit.
In our current age of human gene editing, many researchers believe they can cure sickle cell disease using techniques like CRISPR. But in light of its evolutionary role, it may be pertinent to ask—should we?
Understanding genetics of sickle cell anemia
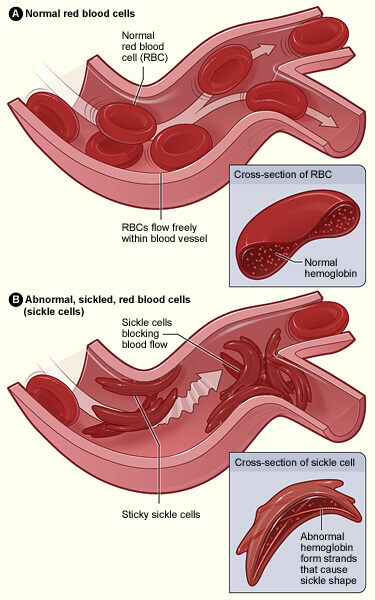 Sickle cell disease is a simple condition. It is caused by just one typo in just one gene. But that gene is very important; it codes for hemoglobin which, while inside red blood cells, carries vital oxygen around to all the cells in our bodies.
Sickle cell disease is a simple condition. It is caused by just one typo in just one gene. But that gene is very important; it codes for hemoglobin which, while inside red blood cells, carries vital oxygen around to all the cells in our bodies.
Everyone inherits two copies of the hemoglobin gene: one maternal and one paternal. But only a person with two copies of the sickle cell version of the gene (called an allele) has the disease. The mutated hemoglobin causes red blood cells to have a ‘sickle shape’ and the hemoglobin’s ability to carry oxygen is diminished. These misshapen cells are inflexible and can block blood vessels impairing blood flow and cause subsequent anemia.
Despite these effects, the gene is extremely common in the human gene pool. Five percent of the human population carries it and in some places, that percentage is even higher. In places where malaria is endemic, such as West Africa, 25 percent of the population carry at least one copy.
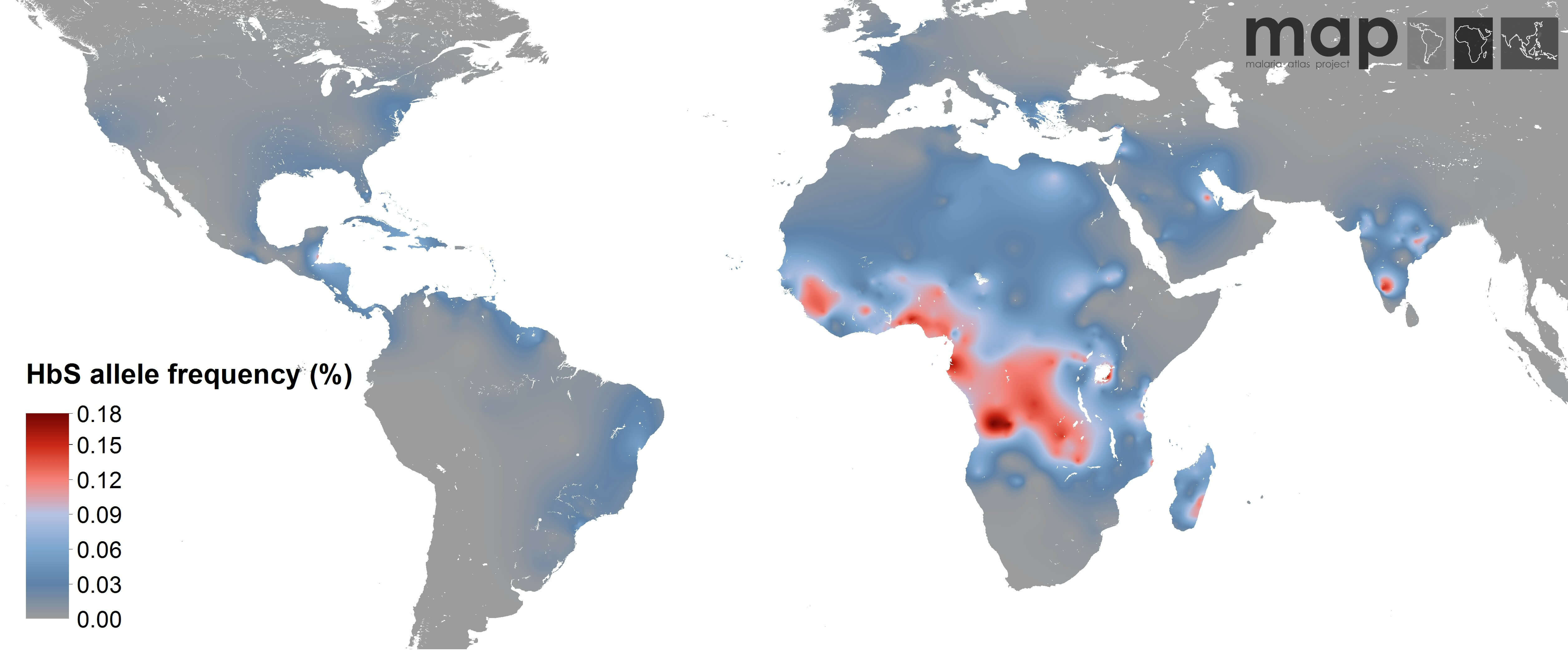
The above map, of the distribution among humans of the sickle cell allele, trends very well with areas where malaria is a major health problem. According to the World Health Organization, there were 214 million cases, most in Africa. The WHO also estimates that 3.2 billion people (almost half of planet Earth’s population) are at risk for malaria.
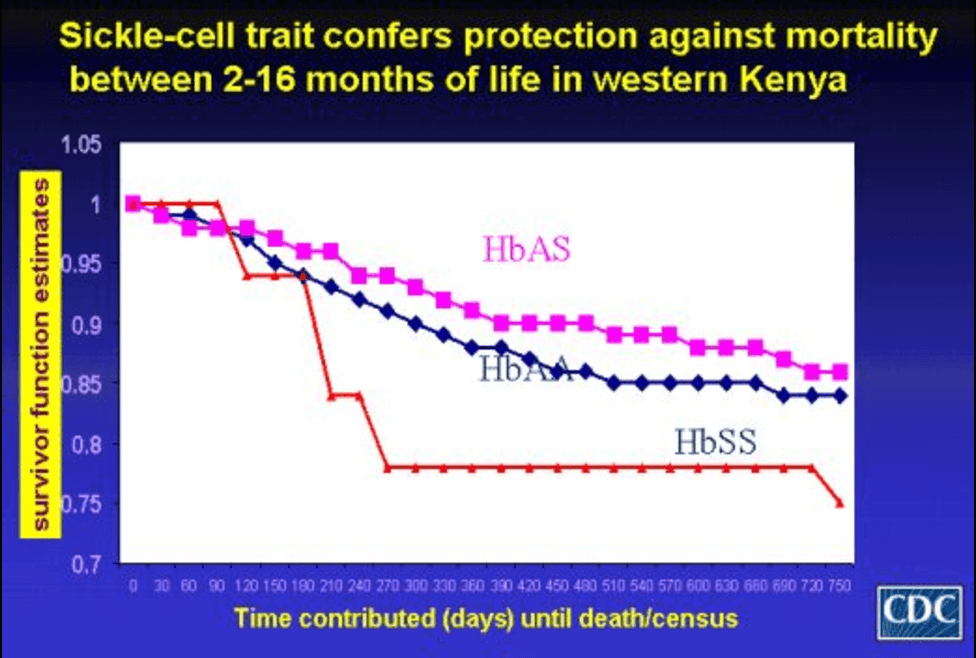
While the mechanisms are still being worked out, it appears that the blood of anyone with a sickle cell allele is inhospitable for the parasite, reducing the likelihood that it causes the disease. A 2005 study found that having the allele was “50% protective against mild clinical malaria, 75% protective against admission to the hospital for malaria, and almost 90% protective against severe or complicated malaria.”
Sickle cell disease isn’t the only disease causing gene that also appears to offer carriers a genetic inoculation. The cystic fibrosis (CF) gene, which codes for an important cell membrane protein that is particularly important for lung function, is thought to protect carriers against infection by the tuberculosis bacterium. It is thought that lacking a functional version of the CF gene starves the organism because a byproduct of the functional gene is needed to construct its cell wall. The CF gene is found in 2 percent of people with European ancestry, a region where tuberculosis is still a problem. The Tay-Sachs gene appears to serve a similar purpose among Ashkenazi Jews. Researchers believe many other similar relationships exist.
To edit or not to edit
These conditions may put clinicians in a peculiar place. If they use CRISPR to correct these faulty genes and cure the disease, patients will then be susceptible to these other diseases. Rob Peter to pay Paul. And scientists are close to being able to do this.
In the last few months, two teams of scientists in California, using the powerful gene editing technique known as CRISPR, have taken giant leaps forward in curing sickle cell disease. In two recent publications, one in Nature and one in Science Translational Medicine, researchers reported successfully editing the genome of mice cells to remove the disease-causing gene.
The results, according to both research teams, are robust enough to justify beginning human clinical trials. “What we’ve finally shown is that we can do it. It’s not just on the chalkboard,” said Dr. Matthew Porteus, senior author of the Nature study. “We think we have a complete data set to present to the FDA to say we’ve done all pre-clinical experiments to show this is ready for a clinical trial,” Porteus told Reuters.
In places such as Europe, America, Canada and northern Asia, this really shouldn’t be much of a problem because malaria is so uncommon that the risk of contracting it is too low to warrant concern. But in endemic regions, such as Africa and South America, there may be some pause for caution. Malaria is so common and so deadly—500,000 annual fatalities—that there may need to be some debate on the merits and risks of stripping people of their genetic shield. It may be that until we have malaria under control—either by a vaccine, more efficient cure or GM mosquitoes that reduce the wild population of the parasite’s vector—people in these regions shouldn’t have these protective genes removed.
One solution to this problem, at least for conditions which require two copies of the gene to manifest, is to only edit-out one copy of the gene. This gives a person the best of both worlds: no sickle cell disease and malaria resistance. However, CRISPR can’t do this—it’s all or nothing, at least for now.
But when the day comes that CRISPR can edit just one copy, we may need to answer a slightly different question: Should we all have one copy of the sickle-cell gene added to our genome?
Nicholas Staropoli is the director of the Epigenetics Literacy Project. He has an M.A. in biology from DePaul University and a B.S. in biomedical sciences from Marist College. Follow him on Twitter @NickfrmBoston.































